


Atoms and the Definition of ElectricityWe know that molecules exist, as some can be seen through an electron microscope. They look like clusters of yet smaller particles.But if the molecule is the smallest point that we can break our (example) water down to, and it still retain its basic properties (remain water), how can it have smaller particles? If we take water, and break it down through electrolysis, we find that water is made up of 2 chemicals or ELEMENTS : hydrogen and oxygen. 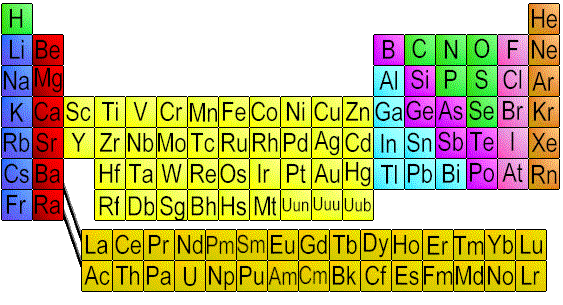 Elements are arranged by their basic properties, as being metals, etc. on a
chart known as the periodic table of the elements. When I went to school, all
matter, regardless of size or state, could be broken down into approximately 105
different elements. Since then, more elements have been discovered, and
will continue to be discovered.
Elements are arranged by their basic properties, as being metals, etc. on a
chart known as the periodic table of the elements. When I went to school, all
matter, regardless of size or state, could be broken down into approximately 105
different elements. Since then, more elements have been discovered, and
will continue to be discovered.
Some of the more common elements are carbon, copper, oxygen and aluminum. Elements may exist alone, or they may exist in clusters, or molecules, along with other elements. For example, a piece of copper wire is solely made up of the element copper. By comparison, water is a combination of two different elements: oxygen and hydrogen. An element can be broken down into even smaller particles, called atoms. An atom is the smallest unit into which an element can be broken down and still retain its original characteristics. An atom resembles a little solar system. The center of this solar system, called the NUCLEUS , is made up of parts known as PROTONS and NEUTRONS . Around the NUCLEUS, tiny little particles are constantly rotating in an orbit. We call these particles ELECTRONS 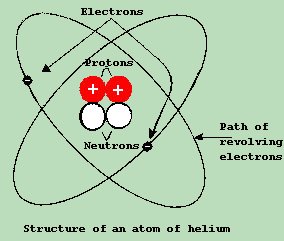
Fig. 1-1 illustrates and atom of helium. Note that it contains 2 electrons, 2 protons and 2 neutrons. The atom is far too small to be seen, even with the aid of the most powerful microscope. However, we do have a vast amount of knowledge about the atom and its inner parts. The proton differs both electrically and physically. Electrically, the proton is POSITIVELY CHARGED , and is about 1850 times heavier than the electron. The orbiting electron, on the other hand, is much lighter, and is said to be NEGATIVELY CHARGED . Note the fact that the larger mass / heavier particle is in the center, and the lighter / smaller mass particle is orbiting it. This is a common feature in nature. Think of a planet, and its moons. The smaller, lighter moons orbit the larger, heavier planet. For the sake of simplicity, the neutron can "effectively" be thought of as consisting of both a proton and an electron. It has the same approximate weight as the proton, however, it is neutral in charge. This is because the positive charge of the proton cancels out the negative charge of the electron. Now atoms are not always so simple as the Helium atom discussed above. They always have the same parts, but not always in the same amounts or configurations. Atoms with more protons and electrons, of course, must be larger and heavier. Under normal circumstances, Atoms seek to be neutral in charge, and so will have an equal amount of electrons and protons. So if an atom like copper, has 29 protons in its center, it will also have 29 electrons. Because these electrons are rotating in an orbit, having too many electrons in a given orbit could cause them to crash into each other. So mother nature placed them in different orbits on different levels. We call them layers or rings. Depending on which ring we are discussing, each ring has a maximum amount of electrons which it can hold, without having to form another ring. For instance, the first ring can only hold 2 electrons. So if we have an atom with 3 protons, (As in the case of Lithium) it must also have 3 electrons. Since it can only hold 2 electrons in the number 1 ring, it is forced to create a second ring, with only 1 electron in it. In the case of Lithium, this 1 electron is said to exist in the outer ring, or the VALENCE RING In Electronics, we are mainly concerned with this VALENCE RING, because it is here that the magic of Electronics takes place. If a given ring is shy of being full, it wants to 'borrow' an electron from somewhere else. If an atom has one too many electrons, it pushes the 'extra' electron way out on a ring of it's own, and tries to 'loan' it to another atom. Electronics, in its purest form, is the study of the movement of electrons from one atom to another. Usually, this takes place by borrowing and loaning (temporarily) of electrons. While we can not actually see this going on, we can monitor it's effects, which can be amazing! The Law of Electromagnetic charges:Most objects, such as a piece of cork normally have a neutral or zero charge; that is, they contain as many electrons as they do protons. If a piece of cork could be made to have an excess of electrons, it would become negatively charged. On the other hand, if the cork were to be made to have a deficiency of electrons, then we would have an excess of protons, and it would then be positively charged.If we take any positively charged body, and bring it near a negatively charged body, the two bodies will be drawn together. If on the other hand, the two objects have like charges, then they will repel each other. These two reactions form the basis of the first law of electricity, known as The Law of Electromagnetic Charges. Differences of Potential:If we connect a copper wire between two oppositely charged bodies, an electron flow would result. Electrons will flow from the negatively charged body to the positively charged body. This is because it is a basic law of nature that CHARGED BODIES SEEK TO BECOME NEUTRAL . What happens here, is that the positively charged body,which has a deficiency of electrons, attracts the excess of electrons from the negatively charged body. This action continues until the deficiency and excess of electrons disappeared and the two bodies become neutral. DIFFERENCE OF POTENTIAL between them. We call this difference of potential ELECTRICAL PRESSURE , or VOLTAGE . |
| (On The Following Indicator... PURPLE will indicate your current location) | ||||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 |
| 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 |
| 51 | 52 | 53 | 54 | 55 | 56 | 57 | 58 | 59 | 60 | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 | 71 | 72 | 73 | 74 | 75 |
| Otherwise - please click to visit an advertiser so they know you saw their ad! |
